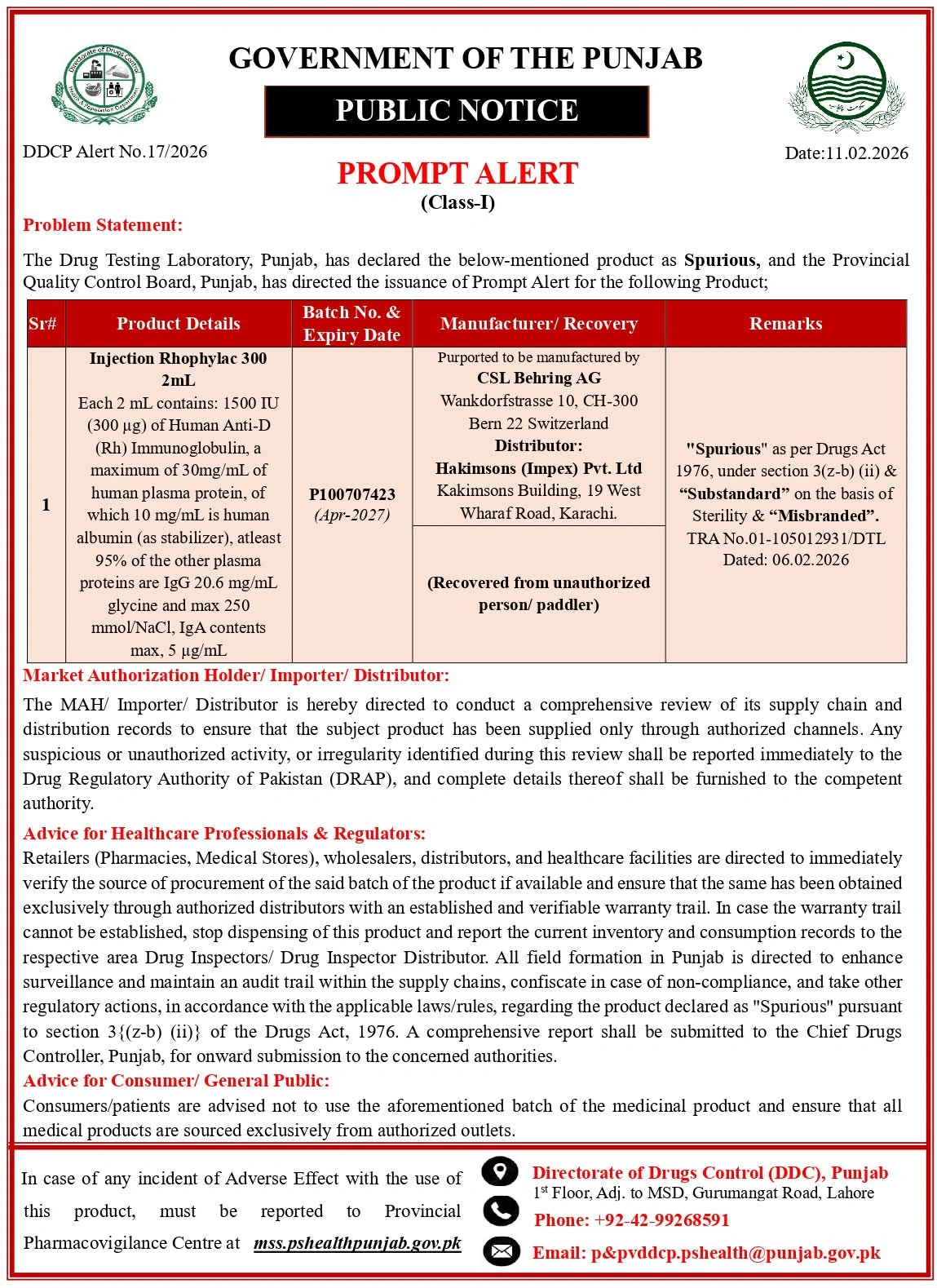
Drug Testing Laboratory Punjab Declares Injection Rhophylac 300 Spurious; Provincial Quality Control Board Punjab Issues Prompt Alert
(Publish from Houston Texas USA)
(Muhammad Mansoor Mumtaz Lahore)
Punjab Drug Testing Laboratory flags Injection Rhophylac 300 as counterfeit; authorities warn public to avoid use.
The Drug Testing Laboratory, Punjab, has declared the product mentioned below as spurious, and the Provincial Quality Control Board, Punjab, has directed the issuance of a prompt alert for the following product. Injection Rhophylac 300 2mL, Each 2 mL contains: 1500 IU (300 ug) of Human Anti-D (Rh) Immunoglobulin, a maximum of 30mg/mL of human plasma protein, of which 10 mg/mL is human albumin (as stabilizer), at least 95% of the other plasma proteins are IgG 20.6 mg/mL glycine and max 250 mmol/NaCl, IgA contents max, 5 ug/mL.
Market authorisation holder/importer/distributor
The MAH/importer/distributor is hereby directed to conduct a comprehensive review of its supply chain and distribution records to ensure that the subject product has been supplied only through authorised channels. Any suspicious or unauthorised activity or irregularity identified during this review shall be reported immediately to the Drug Regulatory Authority of Pakistan (DRAP), and complete details thereof shall be furnished to the competent authority.
Advice for healthcare professionals and regulators
Retailers (pharmacies, medical stores), wholesalers, distributors and healthcare facilities are directed to immediately verify the source of procurement of the said batch of the product, if available, and ensure that it has been obtained exclusively through authorised distributors with an established and verifiable warranty trail. If the warranty trail cannot be established, stop dispensing this product and report the current inventory and consumption records to the respective area drug inspectors and the distributor. All field formation in Punjab is directed to enhance surveillance and maintain an audit trail within the supply chains, confiscate in case of non-compliance, and take other regulatory actions, in accordance with the applicable laws/rules, regarding the product declared as ‘Spurious’ pursuant to section 3{(z-b) (ii)} of the Drugs Act, 1976. A comprehensive report shall be submitted to the Chief Drugs Controller (Punjab), for onward submission to the concerned authorities.
Advice for consumers and the public
Consumers/patients are advised not to use the aforementioned batch of the medicinal product and ensure that all medical products are sourced exclusively from authorised outlets.
For more information please visit our National news.
